Novo Nordisk's latest Phase II clinical data show that the single molecule GLP-1+amylin dual agonist Amycretin has shown positive effects in obese and type 2 diabetes patients.
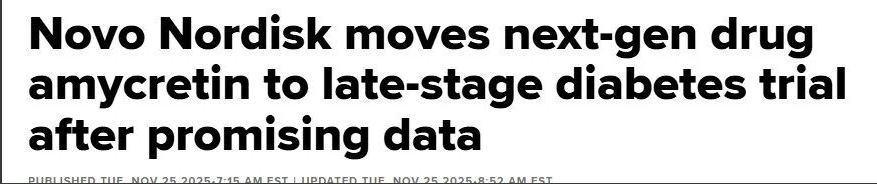


Basic introduction Chinese Name: glucagon like peptide (GLP-1) and amylin receptor agonist polypeptide English Name: amycretin/zenagamtide Company No.: gt-l020/gt-m16083cas No.: 3005889-81-3sequence:H-His-{Aib}-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Val-Ser-Ser-Tyr-Leu-Glu-Glu-Gln-Ala-Ala-Arg-Glu-Phe-Ile-Ala-Trp-Leu-Val-Arg-Gly-Arg-{Lys(diacid-C18-γ-Glu-AEEA-AEEA)}-Gly-Gly-Gly-Gly-Glu-Ala-Ser-Glu-Leu-Ser-Thr-Ala-Ala-Leu-Gly-Arg-Leu-Ser-Ala-Glu-Leu-His-Glu-Leu-Ala-Thr-Leu-Pro-Arg-Thr-Glu-Thr-Gly-Ser-Gly-Ser-Pro-NH2 Molecular formula: C343H50N94O116molecular weight: 7846.5753
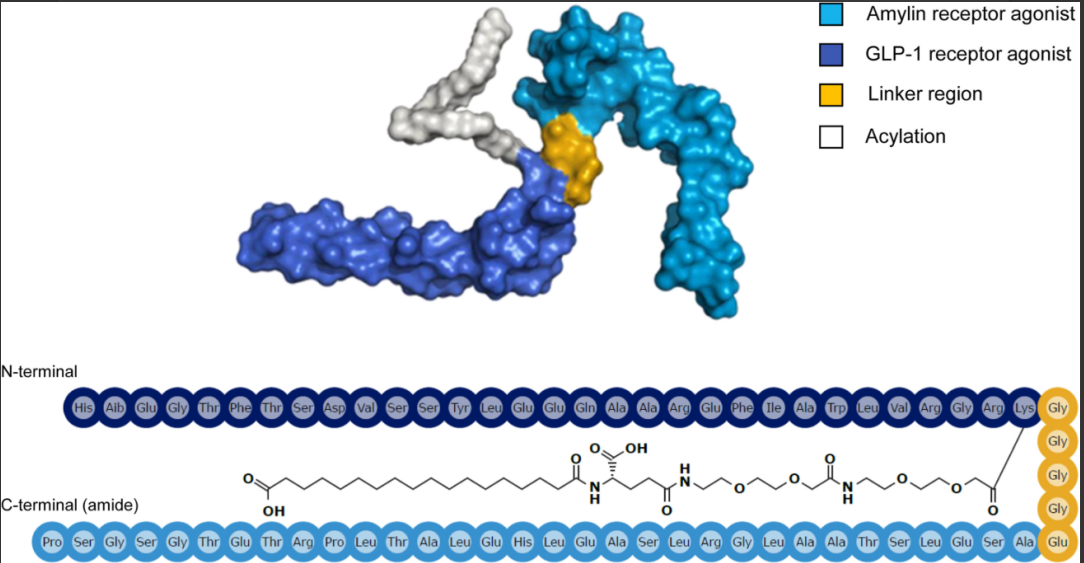
Chemical structure formula of Amycretin
Weight loss potential
Subcutaneous injection once a week, weight loss of up to 14.5% in 36 weeks, and no curative effect plateau was observed.
Highlights of oral preparations
Daily oral administration of amycretin also showed good effects, with HbA1c decreasing as high as 1.5%.
If further validation is successful, it is expected to become another oral GLP-1 drug that can be used for weight loss after smeglutide oral agent, providing more flexible options for patients.
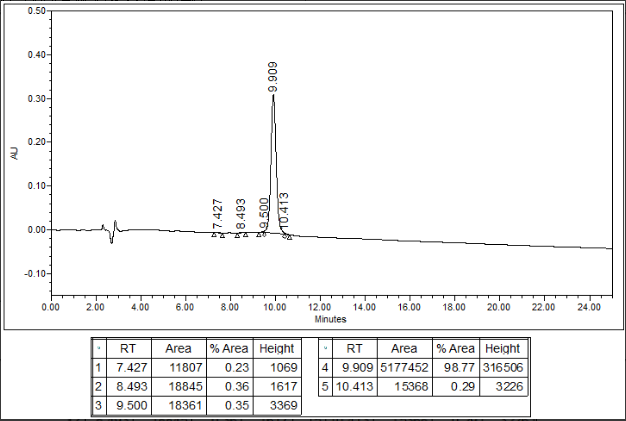
Amycretin HPLC
Safety observation
The current clinical data show that the safety is consistent with the existing glp-1/ amylin therapy, mainly with mild to moderate gastrointestinal reactions.
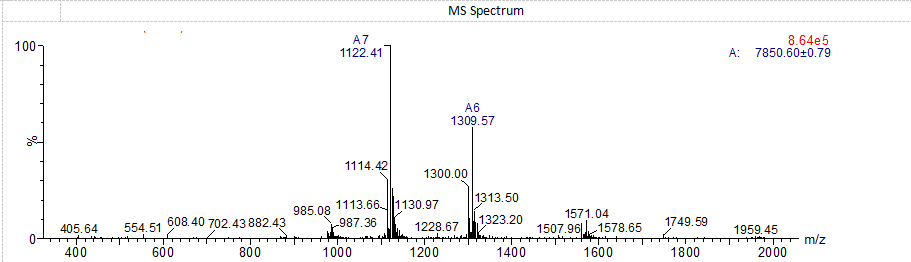
Amycretin MS
Clinical significance
This is the first systematic evaluation of amycretin in patients with type 2 diabetes mellitus. The results show that its weight loss effect is significant and the potential of oral + injection dual-mode flexibility.
If the follow-up clinical validation is successful, it is expected to enter phase 3 clinical trials.
Disclaimer: This article is an industry exchange study. The copyright belongs to the original author. If there is infringement, you can contact to delete it
Post time: 2025-12-01
