I. The Birth and Development of Enfuvirtide Acetate
Enfuvirtide acetate is an anti-AIDS drug jointly developed by the Swiss company Roche and the American company Trimeris. Its emergence has brought new hope to AIDS treatment. In 2003 and 2000, this drug was approved by the US FDA for the treatment of AIDS in adults and children aged 6 and above.
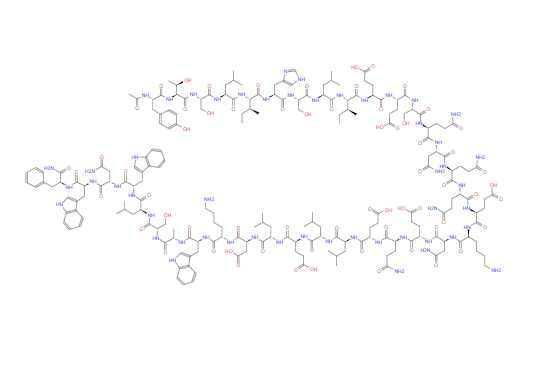
Enfuvirtide acetate is a linear 36-amino-acid polypeptide chain synthesized from amino acids, featuring a unique acetylation structure and carboxamide structure. It binds to the carboxamide structure. The triple helix of the HR1 structural region, which is coiled and spiral, combines to block the formation of the endogenous hexameric bundle, thereby inhibiting the fusion of the virus.
Through clinical trials, enfuvirtide acetate has been proven to be a safe and effective therapeutic drug. During the treatment process, only mild or moderate side effects occurred at the injection site. This characteristic has enabled it to play a significant role in the field of AIDS treatment.
As the research progressed, the mechanism of action of enfuvirtide acetate was also better understood. As a fusion inhibitor, it interferes with the entry of HIV-1 into T cells by preventing the contact and fusion between the virus and immune cells, thereby protecting the immune system of AIDS patients from being damaged by the virus. This unique mechanism of action provides new ideas and methods for the treatment of AIDS.
II. Widely used in clinical practice
The combined medication has a remarkable effect.
Efnveldine acetate has a new mechanism of action and can be used in combination with protease inhibitors and protease inhibitors, or reverse transcriptase inhibitors. Using antiviral drugs in combination at different stages can more effectively inhibit the replication of HIV-1. According to research data, after combined use, the inhibition rate of HIV-1 replication can be increased to over 80%. This combined use reduces the occurrence of drug-resistant viruses and is beneficial for maintaining the treatment of patients. For example, when efnveldine acetate is combined with protease inhibitors, it can be used to intervene in different aspects of virus replication of HIV, greatly improving the treatment effect. At the same time, combined use can also reduce the dosage of a single drug and reduce the occurrence of drug side effects.
(2) Safe and effective with minimal side effects
Clinical studies have confirmed that enfuvirtide acetate is a safe and effective drug for treating HIV-1 positive patients in both adults and children. In many clinical trials, only mild or moderate side effects occurred at the injection site. These side effects typically manifest as local swelling, pain, or itching, but generally do not have a significant impact on the patient's health. Enfuvirtide acetate is safer than other anti-AIDS drugs. Its unique mechanism of action makes it more accurate in treating the impact of HIV virus on normal human cells during the treatment process. This characteristic provides a relatively safe and reliable treatment option for AIDS patients and brings new hope to the treatment of AIDS.
In the future, with the deepening of research and development innovation, enfuvirtide acetate is expected to be used in combination with more new drugs, providing more personalized and efficient treatment plans for AIDS patients. At the same time, with the continuous advancement of technology, the production cost of enfuvirtide acetate is expected to decrease, making it more accessible and widespread. It can be foreseen that enfuvirtide acetate will continue to play an important role in the field of AIDS treatment and make greater contributions to the global AIDS prevention and control cause.
Post time: 2025-08-11
