What are amino acids?
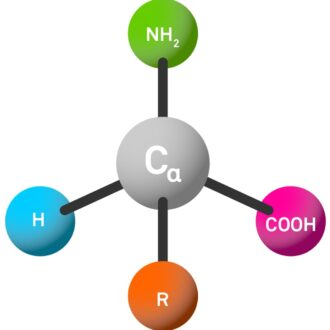
The most common amino acids in nature are called α-amino acids. These molecules have four different substituents attached to the central carbon atom (called the α-C atom):
Amino (NH₂, three-letter code abbreviated as "H-").
Carboxylic acid group (COOH, abbreviated as "-OH" in the three-letter code).
Side chains (R, which are highly variable and determine the properties of amino acids as well as the final peptide).
Hydrogen atom (H).
The connection of α-C atoms to these four different groups gives it unique chemical properties, which play a key role in determining the behavior and properties of amino acids and peptides.
Biological activity of amino acids
Amino acids can exhibit biological activities such as:
Tryptophan (Trp) and glutamic acid (Glu) play a key role in metabolic processes.
The R group (or side chain) determines the unique properties of amino acids. These groups can:
In simple terms: a hydrogen atom like glycine (Gly).
Other acids are included: such as aspartic acid (Asp) and glutamic acid (Glu).
Carry basic groups: arginine (Arg), lysine (Lys) or histidine (His).
Contains polar groups: such as serine (Ser) or threonine (Thr).
Nonpolar hydrocarbons: alanine (Ala), phenylalanine (Phe) or valine (Val).
Sulfur content: as seen in cysteine (Cys) and methionine (Met).
The role of L-amino acids and D-amino acids
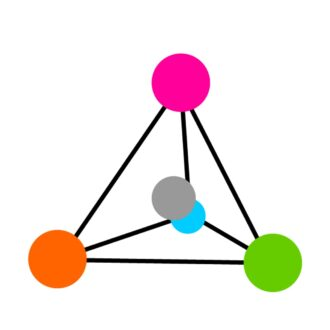
The four substituents of the α-C atom are arranged at the corners of the tetrahedron, with the α-C atom in the center (see Figure 3). This arrangement allows the two forms of amino acid molecules to exist in mirror form, similar to the left and right hands. These mirror forms are known as "stereoisomers" or "enantiomers".
Biological importance of enantiomers
Although enantiomers have nearly identical chemical and physical properties, their biological effects can differ significantly. Molecular shape is critical to its interaction with biological targets. One enantiomer may bind effectively to the target, another may not, or in some cases have a negative effect. In solution, the enantiomer rotates the polarized light plane in the opposite direction.
Post time: 2025-09-05
