Phosphorylation modification plays a crucial role in the life of cells and affects multiple aspects. The phosphorylation of proteins is closely related to various biological processes, such as DNA damage repair, transcriptional regulation, signal transduction, and the regulation of cell apoptosis. Studying phosphorylated proteins and peptides helps to reveal the mechanisms of these processes and deepen our understanding of the essence of life activities. Hong Peptide Biotechnology possesses mature phosphorylation peptide labeling technology. Leveraging its own technological and raw material advantages, we can synthesize peptides containing phosphorylated serine (pSer), phosphorylated threonine (pThr), and phosphorylated tyrosine (pTyr), and provide high-quality peptides with one to five phosphorylation sites.
Overview of Phosphorylation Marking Technology:
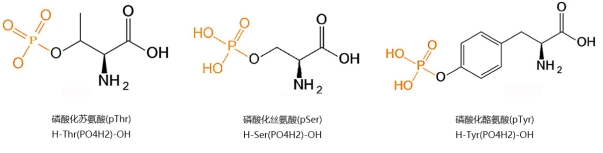
Phosphorylated peptides mainly refer to the side chain hydroxyl groups of serine (Ser), tyrosine (Tyr), and threonine (Thr) residues in the peptide chain being modified into acidic phosphate esters. Phosphorylated peptides play a crucial role in the study of protein phosphorylation. Therefore, it is particularly important to deeply explore the phosphorylation reactions of proteins and peptides and to find mature and simple synthetic methods.
At present, the methods for phosphorylation modification of peptides mainly fall into two categories:
(1) Directly incorporate the appropriately protected phosphorylated amino acids into the peptide sequence:
First, the amino acids that need to be phosphorylated (such as Thr, Ser or Tyr) are subjected to phosphorylation treatment and appropriately protected. Then, following the conventional solid-phase peptide synthesis (SPPS) process, the phosphorylated monomers are linked to the specific sites of the polypeptide. This method is simple to operate and has become one of the main techniques for point phosphorylation modification of peptides.
(2) After synthesizing the polypeptide sequence on the resin, the side chain hydroxyl groups of Ser, Tyr or Thr are phosphorylated:
When performing phosphorylation modification, if the method of directly condensing the phosphorylated monomers into the polypeptide is adopted, the phosphorylated amino acid, due to its larger side chain, causes an increase in steric hindrance, making the condensation with the peptide chain difficult. Moreover, the introduction of subsequent amino acids will also become relatively complex, especially when there are multiple phosphorylation sites. The synthesis process will be extremely difficult, and the final product will be very complex, making separation difficult and the yield extremely low. Therefore, when there are multiple sites requiring phosphorylation in the polypeptide chain, it can be considered to complete the synthesis of the polypeptide sequence on the resin first, and then phosphorylate the side chain hydroxyl groups of Ser, Tyr or Thr. During this process, first selectively remove the side chain protecting groups of the amino acid to be labeled. For Tyr and Thr, the unprotected amino acids of their side chains can be directly reacted. The side chain protecting groups can be quantitatively removed under the condition of 1% TFA/DCM. In this method, the active intermediate of bis-benzyl phosphoramidate and tetrazole can be generated by the reaction with the latter, and it can be linked to the hydroxyl group. Then, an oxidation reaction under peroxide acid conditions can be carried out to generate the phosphonyl group, completing the phosphorylation.
Post time: 2025-07-16
