Peptide-like synthesis technology
The research and development of peptide drugs is growing rapidly in medicine. However, the development of peptide drugs is limited by their own characteristics. For example, due to the special sensitivity to enzymatic hydrolysis, the stability is reduced, and the variability of the steric conformation results in low targeting specificity, low hydrophobicity, and lack of a specific transport system. In order to overcome these peptides, proposed many solutions and successful application of one kind of peptide is one of them.
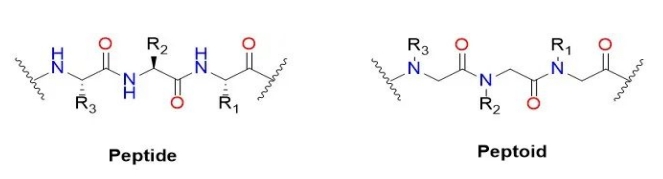
Kind of peptide (English name: Peptoid) or Poly – N – instead of glycine (English name: Poly real – N – substitutedglycine), it is a quasi peptide compounds of peptide in the main chain. The alpha carbon side chain transfers the main chain nitrogen instead of the side chain. In the original polypeptide, the R group of the amino acid side chain represents 20 different amino acids, but the R group has more options in the peptoid. In peptide, peptide on the main chain of amino acids in alpha carbon nitrogen instead of side chain transfer to the main chain. It is worth mentioning that peptides in general do not produce the same high-level ordered structures as secondary structures in peptides and proteins due to the lack of hydrogen on the backbone nitrogen. Peptide initial purpose is to develop stable and protease peptide of small molecule drugs.
Analysis of peptide-like synthesis techniques
The method of peptide synthesis was introduced
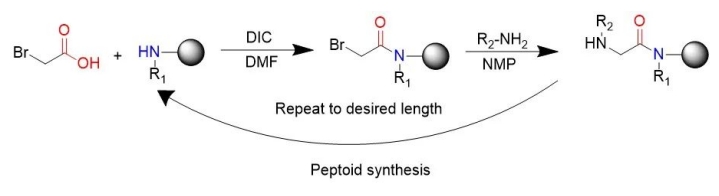
The generally popular peptide-like synthesis method is the subsingle synthesis method invented by RonZuckermann, each of which is divided into two steps: acylation and displacement. In acylation, the first step is to activate the haloacetic acid to react with the amines remaining at the end of the previous step, most commonly diisopropyl carbonized diimine. bromoaceticacid was activated by diisopropylcarbodiimide. “In substitution reactions (bimolecular nucleophilic substitution reactions), an amine, typically primary, attacks the alternative halogen to form an N-substituted glycine.” The subunitary synthetic route uses readily available primary amines to generate peptides, thereby enabling the chemical synthesis of peptides.
Solid extension in class peptides synthesis has the rich experience, can provide you with a variety of kind of peptide synthesis service.
Analysis of peptide-like synthesis techniques
The advantage of such peptide
More stable: peptoids are more stable in vivo than peptides.
More selectivity: Peptoids are well suited for combined drug discovery studies because a large variety of different polypeptide building blocks can be obtained by modification of the backbone amino group.
More efficient: The abundance of peptoid structures can make peptoid a good choice for scanning methodology to quickly find specific structures that bind to proteins.
More market potential: the characteristics of the kind of peptide let it become a kind of drug development has great potential.
Post time: 2025-07-02