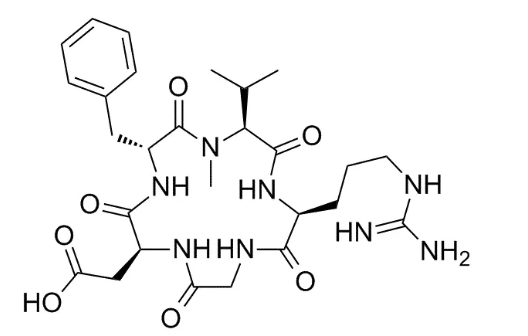
Cilengtitde is developed by Merck, the first new type of tumor targeted therapeutic drugs – integrin inhibitors. Integrin is a cell surface receptor that regulates the dysregulation of a variety of tumor cells, thereby promoting tumor growth, survival and invasion. Integrins also play an important role in tumor angiogenesis.
Cilengtitde can block the binding of integrin to extracellular matrix and inhibit the process of integrin-mediated signaling. This drug plays an anti-tumor role by blocking cell proliferation, survival and migration and inducing endothelial cell apoptosis. In the early studies of glioma (most invasive and difficult to treat tumors), Cilengitide in Dan medicine combined with chemotherapy has made encouraging achievements, has good security, is expected to become new therapy for tumors.
Both αv integrin and epidermal growth factor receptor (EGFR) are widely expressed in NSCLC. Merck CERTO launched phase ii study, explore Cilengitide + cetuximab plus cisplatin/changchun rui marina (or cisplatin/gemcitabine) combination therapy in patients with late NSCC efficacy and safety of first-line treatment.
This is a comparative study on the open multicenter, randomized, To compare the efficacy and safety of two regimens of Cilengitide (2000mg once weekly or 2000mg twice weekly) and cetuximab and platinum-based chemotherapy (cisplatin/vinorelbine or cisplatin/gemcitabine) combined with cetuximab alone and platinum-based chemotherapy in the first-line treatment of advanced non-small cell lung cancer (NSCLC).
Since January 2010, the CERTO study is expected to achieve better results in the treatment of late-stage NSCLC.
Post time: Jun-27-2024