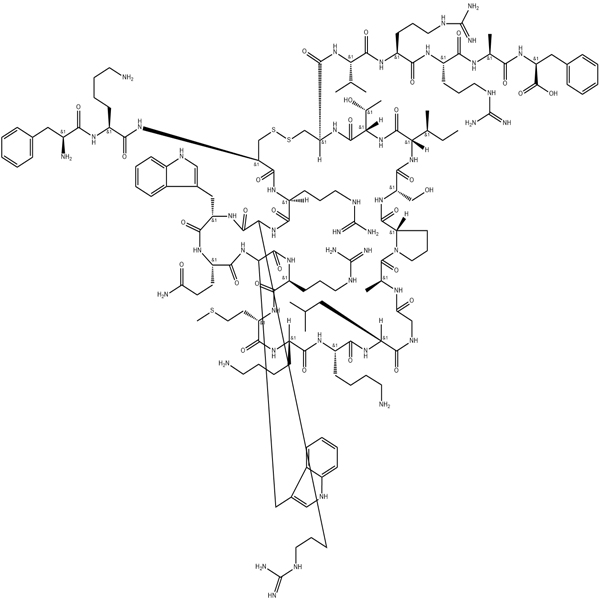
ACTH (1-39) (mouse rat)/77465-10-2/GT Peptide/Peptide Supplier
Description
ACTH 1-39 at concentrations of 100 to 400 nM had no toxic effect on neurons, whereas ACTH prevented excitotoxic neuronal death induced by glutamate (100 μM), NMDA (1 mM), AMPA (50 μM), and kainic acid (25). Micrometer). ACTH at 400 nM provided adequate protection in each case. ACTH at 200 or 400 nM protected neurons from quinolinic acid (25 μM). ACTH also prevented cell death induced by 2 μM H 2 O 2, resulting in the generation of reactive oxygen species (ROS), with a significantly stronger protective effect at 400 nM compared to 200 nM. ACTH provided moderate protection against the rapid release of nitric oxide (NO) from NOC-12, but did not prevent the slow release of NOC-18. ACTH (200 or 400 nM) protects neurons from the cytotoxic effects of crispsporin (10-20 nM), a classical inducer of cell death through apoptosis. ACTH reduced cell death from 80% to 55%.
Specifications
Apperance: White to off-white powder
Purity(HPLC): ≥98.0%
Single Impurity: ≤2.0%
Acetate Content(HPLC): 5.0%~12.0%
Water Content (Karl Fischer): ≤10.0%
Peptide Content: ≥80.0%
Packing and Shipping: Low temperature, vacuum packing, accurate to mg as required.
How To Order?
1. Contact us directly by phone or email: +86-13735575465, sales1@gotopbio.com.
2. Order online. Please fill out the order online form.
3. Provide peptide name, CAS No. or sequence, purity and modification if required, quantity, etc. we will provide a quotation within 2 hours.
4. Order conformation by duly signed sales contract and NDA(non disclosure agreement) or confidential agreement.
5. We will continuously update the order progress in time.
6. Peptide delivery by DHL, Fedex or others, and HPLC, MS, COA will be provided along with the cargo.
7. Refund policy will be followed if any discrepancy of our quality or service.
8. After-sale service: If our clients have any questions about our peptide during experiment, please feel free to contact us and we will respond to it in a short time.
All products of the company are only used for scientific research purpose, it’s prohibited to be directly used by any individuals on human body.
FAQ:
Which end is best for my research?
By default, the peptide ends with an N-terminal free amino group and a C-terminal free carboxyl group. The peptide sequence often represents the sequence of the mother protein. In order to be closer to the mother protein, the end of the peptide often needs to be closed, that is, n-terminal acetylation and C-terminal amidation. This modification avoids the introduction of excess charge, and also makes it more able to prevent exonucliase action, so that the peptide is more stable.
Which modified labeled polypeptides can be synthesized in Chinese peptide?
Our company provides a variety of modified peptide labeling, such as acetylation, biotin labeling, phosphorylation modification, fluorescence modification, can also be customized according to your special needs.
How do you dissolve polypeptides?
The solubility of polypeptide depends mainly on its primary and secondary structure, the nature of modification label, solvent type and final concentration. If the peptide is insoluble in water, ultrasound can help dissolve it. For basic peptide, it is recommended to dissolve with 10% acetic acid; For acidic peptides, dissolution with 10%NH4HCO3 is recommended. Organic solvents can also be added to insoluble polypeptides. The peptide is dissolved in the least amount of organic solvent (e.g., DMSO, DMF, isopropyl alcohol, methanol, etc.). It is highly recommended that the peptide be dissolved in the organic solvent first and then slowly added to water or other buffer until the desired concentration.
What are the best preservation conditions? How stable is the peptide?
After lyophilized, polypeptide can form fluff or flocculant powder, which can avoid premature degradation of polypeptide. Recommended storage conditions: a. -20℃ storage or dry environment b. Try to avoid repeated freeze-thaw c. Try to avoid storage in solution state (freeze-dried powder can be stored in separate packages for convenience of use) d. If it must be stored in solution, it is recommended to dissolve the peptides in sterile water under weakly acidic conditions and store at -20℃.
How is my peptide transported? What test reports are provided?
All freeze-dried polypeptides are usually stored in special containers of 2 ml or 10ml with original analytical data and synthesis reports containing important information such as sequence, molecular weight, purity, weight, and number of the polypeptide.
What is net weight? What is peptide content?
After lyophilized peptide is generally fluffy and fluff-like, it may still contain trace amounts of water, adsorbed solvents and salts due to the characteristics of peptide itself. This does not mean that the purity of the peptide is not enough, but that the actual content of the peptide is reduced by 10% to 30%. The net weight of the peptide is the actual weight of the peptide minus the water and protonated ions. In order to ensure the concentration of peptide, the non-peptide substances need to be removed from the crude peptide.
How pure can the peptide be?
Our company can provide different purity levels for customers to choose from, from crude to > 99.9% purity. According to customer needs we can provide purity > 99.9% ultra-pure polypeptide.