Fluorescence resonance energy transfer (FRET)
Fluorescence resonance energy transfer (FRET) is a non-radiative energy transfer process in which the donor excited state energy is transferred to the acceptor excited state through the interaction of intermolecular electric couples. This process does not involve photons and is therefore non-radiative. This assay has the advantages of being fast, sensitive and simple.
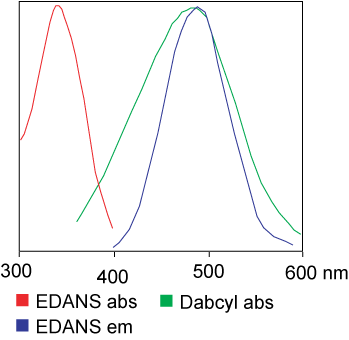
The dye used in the FRET assay can be identical. But in most applications, different dyes are actually used. In brief, the transfer of luminous resonance energy is the transfer of a pair of dipoles from the donor (dye 1) to the acceptor (dye 2) when the donor group is excited. In general, the emission spectrum of the Donor fluorophore group overlaps with the absorption spectrum of the Acceptor group. “When the distance between the two fluorophores is appropriate (10 — 100 A), the transfer of fluorophore energy from the donor to the acceptor can be observed.” The method of energy transfer depends on the chemical structure of the receptor:
1. Is converted into molecular vibration, that is, the luminous light of energy transfer disappears. (The receptor is a light quencher)
2. The emission is more intense than the receptor itself, resulting in a redshift in the secondary fluorescence spectrum.” (Receptors are luminous emitters).
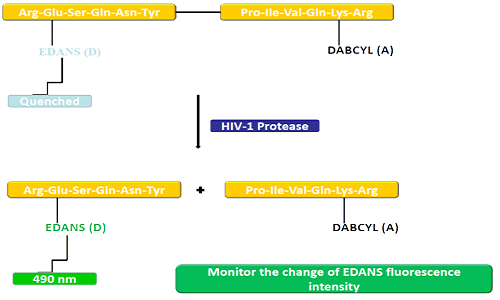
The donor group (EDANS) and acceptor gene (DABCYL) are uniformly linked to the natural substrate of HIV protease, and when the substrate is not disconnected, DABCYL can quenle EDANS and then become undetectable to fluorine. Upon HIV-1 protease disconnection, EDANS is no longer quenched by DABCYL, and EDANS luciferases can subsequently be detected. The availability of protease inhibitors can be monitored by changes in the fluorescence intensity of EDANS.
FRET peptides are convenient tools to study peptidase nonspecificity. Since its reaction process can be continuously monitored, it provides a convenient method for detecting enzyme activity. The sheen produced after the hydrolysis of peptide bonds by the donor/acceptor provides a measure of enzyme activity at nanomolar concentrations. When the FRET peptide is intact, it shows a sudden disappearance of the internal flash, but when any peptide bond opposite the donor/acceptor breaks, it releases a flash, which can be detected continuously and the enzyme activity can then be quantified.
Post time: Aug-14-2023